Every week, the editors of Cancer Today magazine bring you the top news for cancer patients from around the internet. Stay up to date with the latest in cancer research and care by subscribing to our e-newsletter.
Immunotherapies Extend Progression-free Survival as First-line Treatment for Advanced Endometrial Cancer
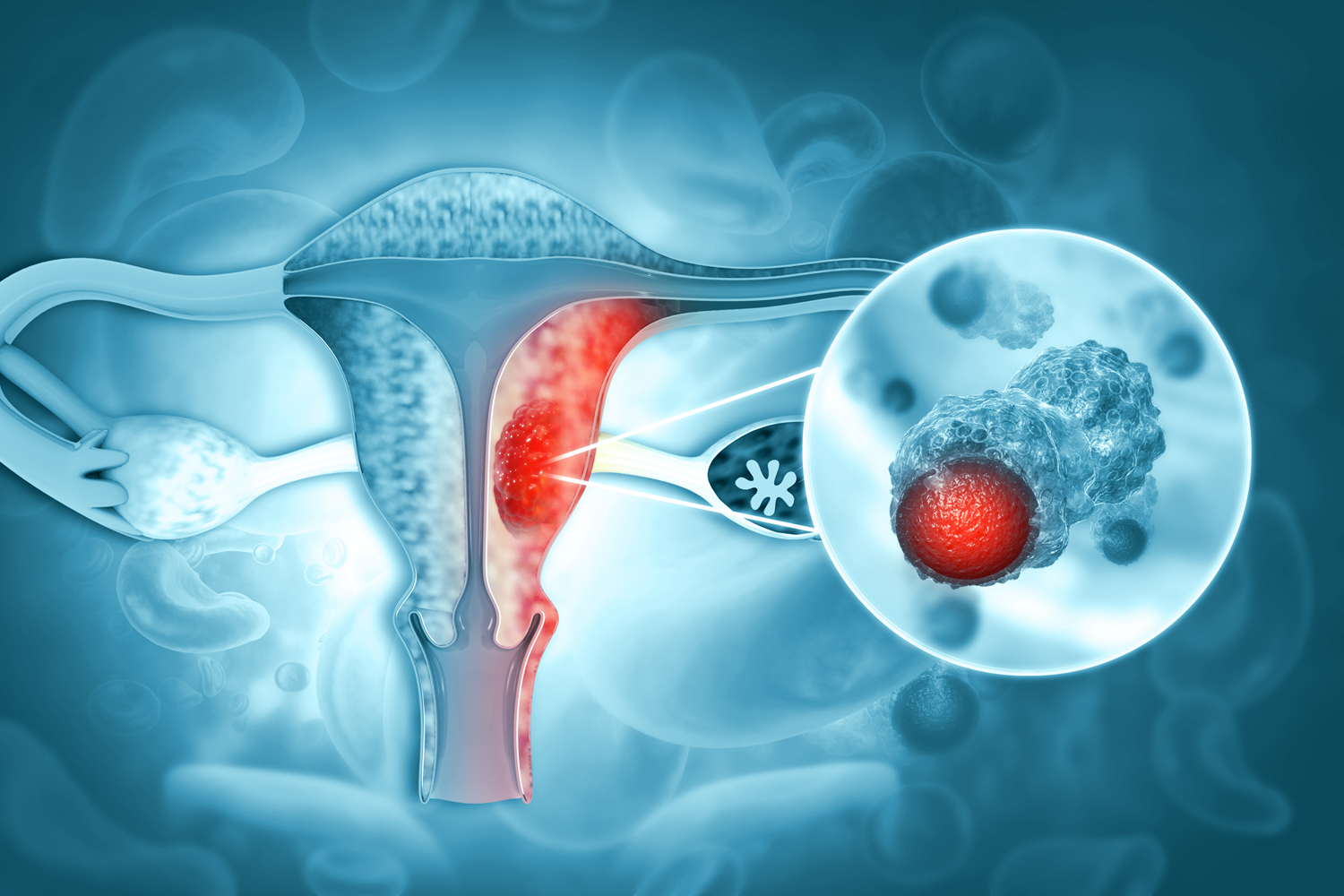
Two studies presented March 27 at the annual meeting of the Society of Gynecologic Oncology (SGO) could pave the way for immunotherapy as first-line treatment for advanced or recurrent endometrial cancer. Keytruda (pembrolizumab) and Jemperli (dostarlimab) are immune checkpoint inhibitors that have previously been approved in combination treatments for advanced or recurrent endometrial cancer after chemotherapy. In new studies presented at the SGO meeting, they were given with chemotherapies in the first line of treatment for advanced or recurrent disease. In both trials, immunotherapy was found to extend the time people went without cancer progressing, though the improvement was even greater for people whose cancer had certain biomarkers, OBR Oncology reported. In the NRG GY018/Keynote-068 trial, people with mismatch repair deficient disease who were given the Keytruda-chemotherapy combination had a progression-free survival of 74% at one year, compared with 38% of those given chemotherapy and a placebo. Results from the RUBY trial found that among people with mismatch repair deficient/microsatellite instability-high disease, 61.4% on the Jemperli-chemotherapy combination went two years without disease progression compared with 15.7% in the chemotherapy and placebo group. NBC News reports that the Food and Drug Administration will have to amend its guidance on immunotherapy before these indications go into wide use, but that regulators are already reviewing these findings. “This is going to drastically change the conversation” David O’Malley, a gynecologic oncologist with the Ohio State University Comprehensive Cancer Center, told NBC News.
Millions Expected to Lose Medicaid Coverage With the End of Pandemic Protection
Starting Saturday, April 1, states will begin to cut what’s expected to total 15 million people with low incomes from Medicaid programs across the U.S. The federal government is ending a temporary guarantee, enacted in response to the COVID-19 pandemic, that allowed people who were on Medicaid to stay on the program without having to prove their continued eligibility. Five states beginning the process April 1 will be joined in the next few months by most other states in reviewing eligibility and removing people who no longer qualify for the program. Enrollment in Medicaid grew by a third over the pandemic, and 85 million people now rely on the program for health coverage. Daniel Tsai, Medicaid director at the Centers for Medicare and Medicaid Services told the Washington Post that the department has been working for months to try to forestall potential problems. Tsai said they want to minimize eligible people losing coverage for technical reasons and to guide people who may no longer be eligible for Medicaid to other insurance coverage. But there will be a lot of people asked to prove their eligibility who have not needed to navigate a renewal in three years and a shortage of employees to help. “We go to sleep at night thinking about this and wake up in the morning thinking about this,” Tsai told the Post. An analysis from the Department of Health and Human Services predicts that 15 million people will lose their coverage, of whom 6.8 million could still be eligible for the program. The number of people expected to lose coverage is particularly large in states that have not expanded coverage under the Affordable Care Act and those that have not chosen to allow benefits for pregnant women to extend for the full year after they give birth.
Lumpectomy Found Safe for People With Multiple Breast Tumors
A study presented at the International Conference on Surgical Cancer Care and published March 28 in the Journal of Clinical Oncology found that some women with multiple tumors in the same breast can safely get treated with lumpectomy, also called breast-conserving surgery. The phase II trial found that women with multiple tumor sites treated with lumpectomy had a 3.1% rate of cancer returning to the breast at five years, well below the 8% rate that the researchers determined as acceptable based on historic recurrence rates in cases with single tumors. Additionally, 70.6% of patients reported that the cosmetic outcomes at two years were good or excellent, though 7.1% converted to mastectomy due to positive margins in the first surgery. Presenting the findings was surgical oncologist Kari Rosenkranz, an author on the study and a surgical oncologist at Dartmouth Health, in Lebanon, New Hampshire. Rosenkranz told Medscape that the decisions around breast surgery can be complicated, and even when breast conservation is feasible, some women choose mastectomy. She said it is critical to tailor therapy to the goals and priorities of the patient. But breast-conserving surgery is an important option that has a number of benefits that can now be offered to some women in this population. “The reason this is so important is that we know that patients who undergo breast conservation report improved quality of life, self-esteem, and body image, and therefore it’s incumbent on us as surgeons to expand the indications for breast conservation where we can,” Rosenkranz said in the presentation.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.