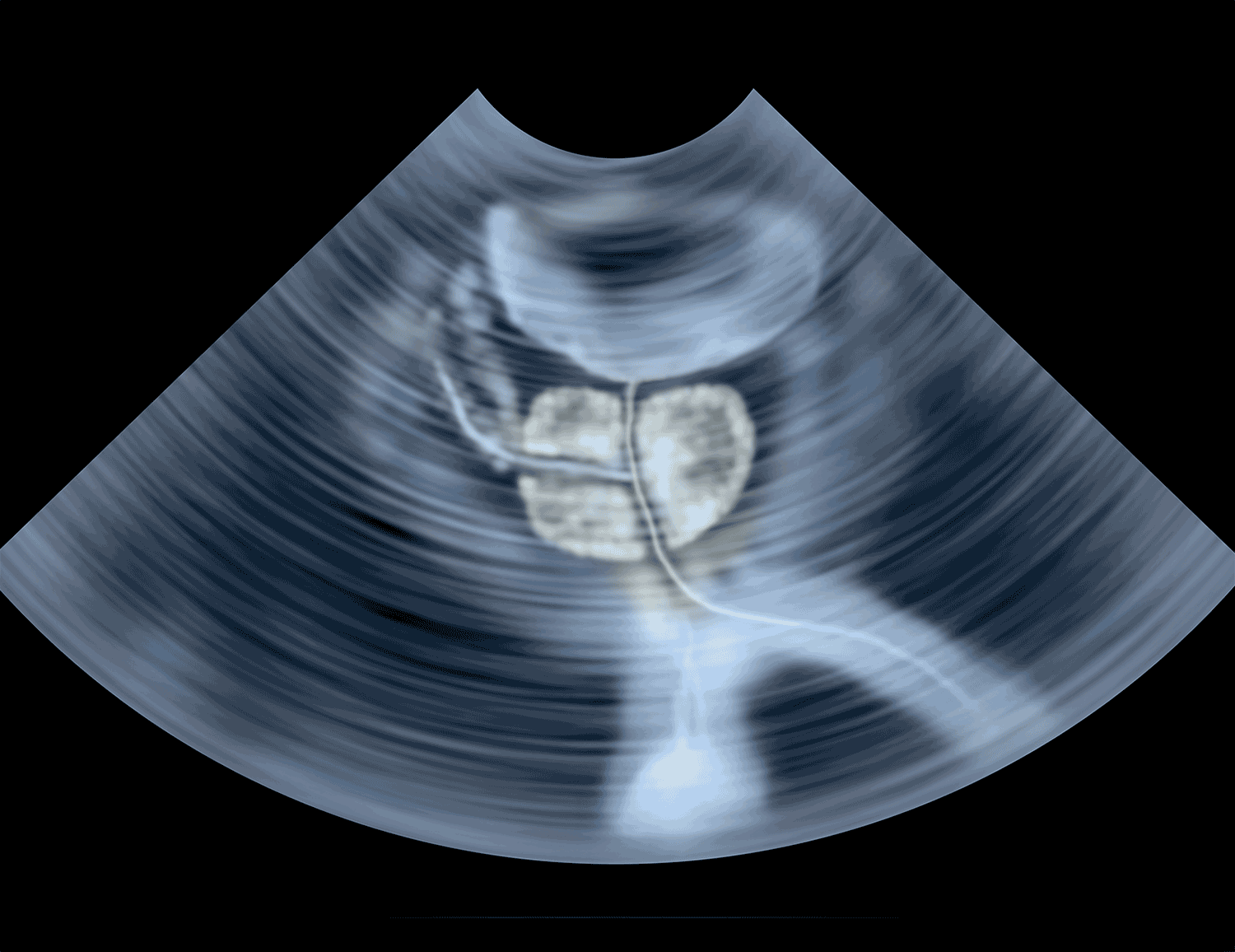
Early Data on Triplet Therapy for Prostate Cancer
The standard of care has shifted over the years for metastatic castration-sensitive prostate cancer—a form of prostate cancer that grows in response to hormones called androgens. The chemotherapy drug docetaxel or new hormonal therapies have been added to the traditional androgen-deprivation therapy (ADT). Early data from a phase III trial presented at the 2021 American Society of Clinical Oncology Annual Meeting, held virtually June 4-8, suggest a combination of all three could extend the time a patient survives without tumor progression based on scans, which researchers call radiographic progression-free survival (rPFS). However, the data are not yet mature enough for results on overall survival to be available, a caveat that was stressed in discussion at the meeting. The PEACE-1 trial, which included 1,173 men with castration-sensitive prostate cancer that was metastatic at diagnosis, showed the triplet therapy of the hormonal drug Zytiga (abiraterone) plus androgen-deprivation therapy (ADT) and docetaxel extended rPFS by a median 2.5 years, when compared with the standard of care, consisting of ADT alone or ADT with docetaxel. The trial could be practice changing, said study investigator Karim Fizazi, a medical oncologist at the Institut Gustave Roussy in Villejuif, France, according to MedPage Today. The discussant for the study, Lisa Horvath, a researcher at the Sydney Cancer Center in Australia, expressed a need for more published results on the approach, and put it in context of similar studies, such as ENZAMET, on which she was a co-investigator. ENZAMET, which looked at Xtandi (enzalutamide), a hormonal therapy, in combination with docetaxel and ADT, showed promising rPFS but did not show improved overall survival, she noted.
FDA Experts Chat about Drug Approvals
After the 2021 American Society of Clinical Oncology Annual Meeting, STAT hosted a virtual live chat with leadership from the Food and Drug Administration (FDA) Oncology Center of Excellence (OCE), including the OCE’s director Rick Pazdur. STAT’s national biotech columnist Adam Feuerstein opened the discussion on June 9 by asking about recent criticism that the FDA’s standards for approval may have become lower, as the FDA approves more drugs at a faster clip. “I think sometimes people don’t understand drug regulation,” said Pazdur. “People want to put regulations that don’t exist on us. For example, we have to demonstrate that the drug is safe and effective, not that it is better than what we approved yesterday. People have to understand that we need to move on from the traditional overall survival endpoint to meet patient needs.” The change is due to a number of factors, including smaller populations of patients who may benefit from targeted therapies, Pazdur noted. Panelists discussed a range of topics, including possible regulatory approval of drugs, such as immune checkpoint inhibitors, from China, the plethora of PD-L1/PD-1 inhibitors, and recent voluntarily withdrawals of immunotherapy indications by pharmaceutical companies. (For more information, read this story published March 23 in Cancer Today.)
Survey Suggests Americans Have Inadequate Knowledge of Palliative Care
A study published June 4 in Cancer Epidemiology, Biomarkers & Prevention finds that American adults report having inadequate knowledge of palliative care. Palliative care strives to improve quality of life for a person who has a serious disease, such as cancer, throughout their illness, but the term is often confused with hospice care, which is provided only at the end of life after active treatment stops. Motolani Ogunsanya, an assistant professor at the University of Oklahoma Health Sciences Center in Oklahoma City, and colleagues assessed knowledge using 3,450 survey responses from the National Cancer Institute’s Health Information National Trends Survey from 2018. Respondents who self-reported their knowledge of palliative care by selecting “I’ve never heard of it” or “I know a little bit about palliative care” were grouped together as having inadequate knowledge, while a response of “I know what palliative care is, and I could explain it to someone else” was considered adequate knowledge. Only 11% of the respondents reported adequate knowledge of palliative care. However, respondents with a prior cancer diagnosis were 51% more likely to have adequate knowledge of palliative care than those who had never been diagnosed with cancer. “If someone is not confident in their knowledge of palliative care, they may be less inclined to ask for it, regardless of how well they do understand it,” said Ogunsanya in a statement from the American Association for Cancer Research (AACR). (The AACR publishes Cancer Today.)
Survey Suggests Americans Have Inadequate Knowledge of Palliative Care A study published June 4 in Cancer Epidemiology, Biomarkers & Prevention finds that American adults report having inadequate knowledge of palliative care. Palliative care strives to improve quality of life for a person who has a serious disease, such as cancer, throughout their illness, but the term is often confused with hospice care, which is provided only at the end of life after active treatment stops. Motolani Ogunsanya, an assistant professor at the University of Oklahoma Health Sciences Center in Oklahoma City, and colleagues assessed knowledge using 3,450 survey responses from the National Cancer Institute’s Health Information National Trends Survey from 2018. Respondents who self-reported their knowledge of palliative care by selecting “I’ve never heard of it” or “I know a little bit about palliative care” were grouped together as having inadequate knowledge, while a response of “I know what palliative care is, and I could explain it to someone else” was considered adequate knowledge. Only 11% of the respondents reported adequate knowledge of palliative care. However, respondents with a prior cancer diagnosis were 51% more likely to have adequate knowledge of palliative care than those who had never been diagnosed with cancer. “If someone is not confident in their knowledge of palliative care, they may be less inclined to ask for it, regardless of how well they do understand it,” said Ogunsanya in a statement from the American Association for Cancer Research (AACR). (The AACR publishes Cancer Today.)
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.