UPDATE: On October 18, 2017, the U.S. Food and Drug Administration approved Kite Pharma’s CAR-T cell therapy for certain types of non-Hodgkin lymphoma. Called Yescarta (axicabtagene ciloleucel), the therapy is priced at $373,000.
In 2010, researchers at the National Cancer Institute (NCI) in Bethesda, Maryland, followed soon by researchers at the University of Pennsylvania in Philadelphia, began publishing encouraging results in patients with advanced blood cancers treated with an experimental form of immunotherapy.
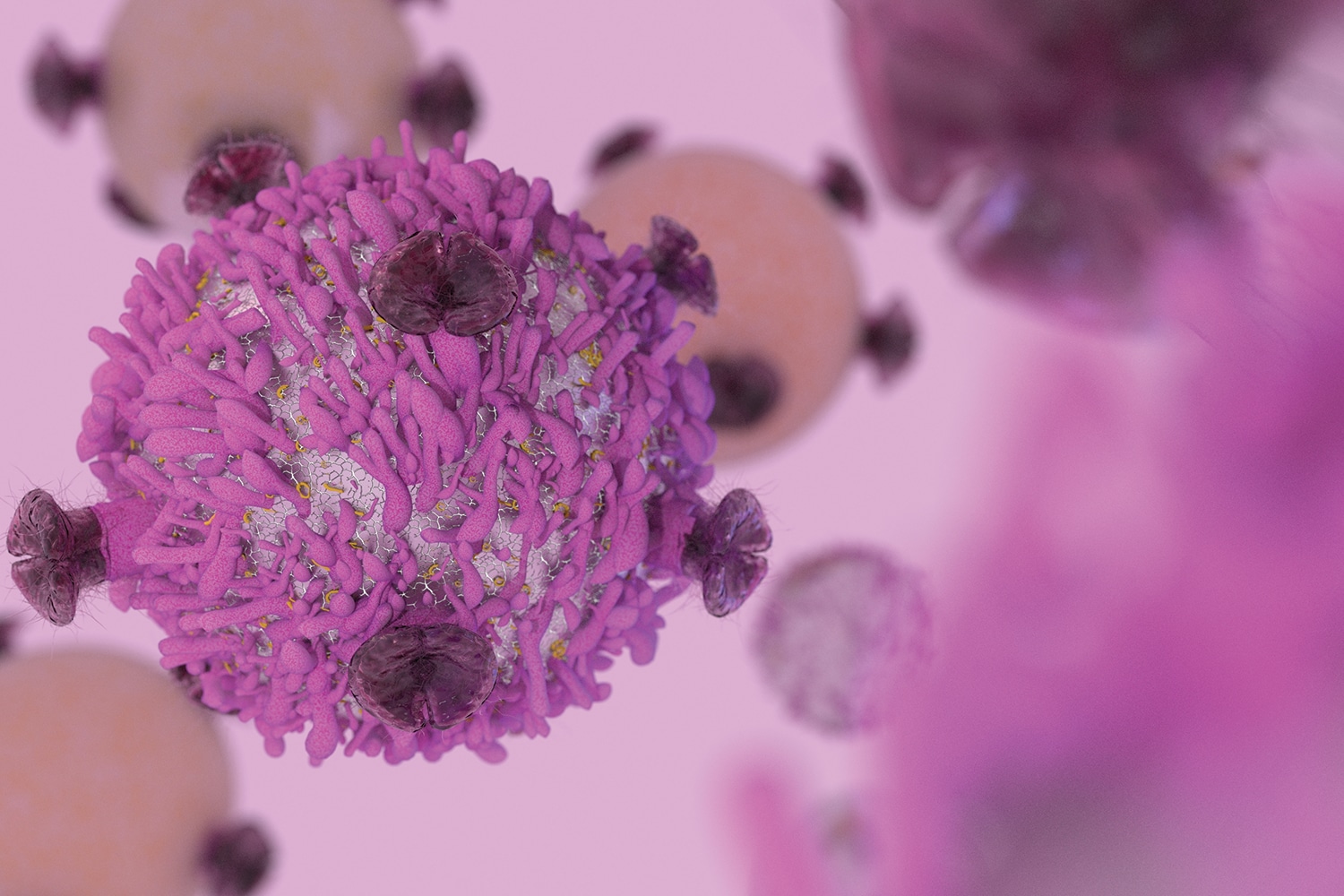
The treatments involve removing T cells, a type of immune cell, from patients’ blood and outfitting the cells with receptors that recognize molecules on the surfaces of cancer cells. The modified cells, referred to as chimeric antigen receptor T (CAR-T) cells, are then infused into the patients, where they track down cancer cells and kill them.
On Aug. 30, 2017, the U.S. Food and Drug Administration (FDA) approved a CAR-T cell therapy for the first time. Kymriah (tisagenlecleucel) was developed by the University of Pennsylvania and Novartis for certain patients ages 25 and under with acute lymphoblastic leukemia (ALL). Another CAR-T therapy, developed by the NCI and Kite Pharma, is for patients with non-Hodgkin lymphoma (NHL) and is being considered for FDA approval.
CAR-T cell therapies work only for a portion of the patients who try them. In a Novartis trial, 52 of 63 pediatric and young adult ALL patients treated with CAR-T cells had a complete response initially. About 64 percent of these patients are expected to still be disease-free after one year. A Kite Pharma trial in 101 patients with certain types of NHL found that about half initially had a complete response and that at six months a little over a third of all patients treated were disease-free.
CAR-T cell treatments have kept some patients cancer-free for years. The first lymphoma patient to have a complete response, treated in 2009, is still in complete remission; the first child with ALL to have a complete response, treated in 2012, also shows no sign of disease.
The treatments come with some safety concerns. Juno Therapeutics ceased development of a CAR-T cell therapy for ALL in adults after five patients died from brain swelling. One patient died from brain swelling in a trial offered by Kite Pharma. Cellectis, a company trying to make a different type of CAR-T cell therapy using modified T cells from healthy donors, had to halt its two trials in September 2017 after a patient death. In the immediate days and weeks following infusion, many patients have symptoms related to a flood of immune molecules in their blood, such as fever and low blood pressure, that can lead to hospitalization.
CAR-T therapies are being studied in other blood cancers, including chronic lymphocytic leukemia, multiple myeloma and acute myeloid leukemia. Using CAR-T cell therapy to treat solid tumors, which make up 80 to 90 percent of cancer cases in the U.S., has proved more challenging. It is difficult to find a molecule to target on the surface of a solid tumor cancer cell that is not also present on essential healthy cells, explains Brian Till, a medical oncologist at the Fred Hutchinson Cancer Research Center in Seattle. Many CAR-T cell therapies in blood cancers kill healthy blood cells as collateral damage, but people can withstand having these cells depleted, says Till, adding, “Those of us using CAR-T cells to treat blood cancers are lucky that we have some good targets to go after.”
Steven Rosenberg, chief of the surgery branch of the NCI, says that treatment of solid tumors is likely to rely on a different kind of modified T cell. Rosenberg, who led the NCI’s CAR-T cell research, is studying a type of modified T cell that resembles naturally occurring T cells that target altered proteins caused by mutations in each patient’s cancer.
For now, Novartis’ newly approved therapy Kymriah will be available at 32 certified medical centers as an option for treatment of the approximately 600 pediatric and young adult ALL patients who relapse annually in the U.S. Treatment with Kymriah has a $475,000 price tag. Assistance will be available to help patients access treatment, Novartis says.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.