A CHECKPOINT INHIBITOR is a type of immunotherapy that works by disabling the “brakes” of the body’s natural defense against cancer, T cells. For many years, progress toward a greater understanding of the mechanisms that underpin these treatments was slow, but there has been a huge uptick over the past decade.
1891: William B. Coley begins treating patients with a rudimentary form of immunotherapy as head of the Bone Tumor Service at Memorial Hospital in New York City. Coley would inject the patients with a mixture of bacteria later known as Coley’s Toxins. Many of his peers discredited his work, and the treatment would fall out of favor alongside the rise of radiation therapy and chemotherapy to treat cancer.
1987: Gene encoding CTLA-4 is discovered.
1992: Gene encoding PD-1 is discovered.
CTLA-4 and PD-1 are negative regulators of T cell immune function, meaning that they serve to suppress the immune system’s response to stimulus. This safeguards against autoimmune disease, but may also undermine the immune system’s ability to destroy cancer cells. Checkpoint inhibitors ensure this doesn’t happen.
2011: The Food and Drug Administration (FDA) approves Yervoy (ipilimumab), a treatment that activates the immune system by blocking CTLA-4, for the treatment of metastatic melanoma.
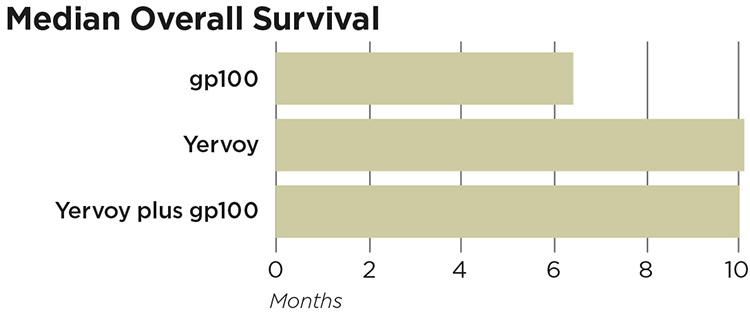
In a randomized clinical trial of 676 patients with metastatic melanoma, 403 received Yervoy plus the gp100 peptide vaccine, 137 received Yervoy alone, and a control group of 136 received gp100 alone. Yervoy alone and combined with the gp100 peptide vaccine improved overall survival compared to treatment with gp100 alone in metastatic melanoma patients.
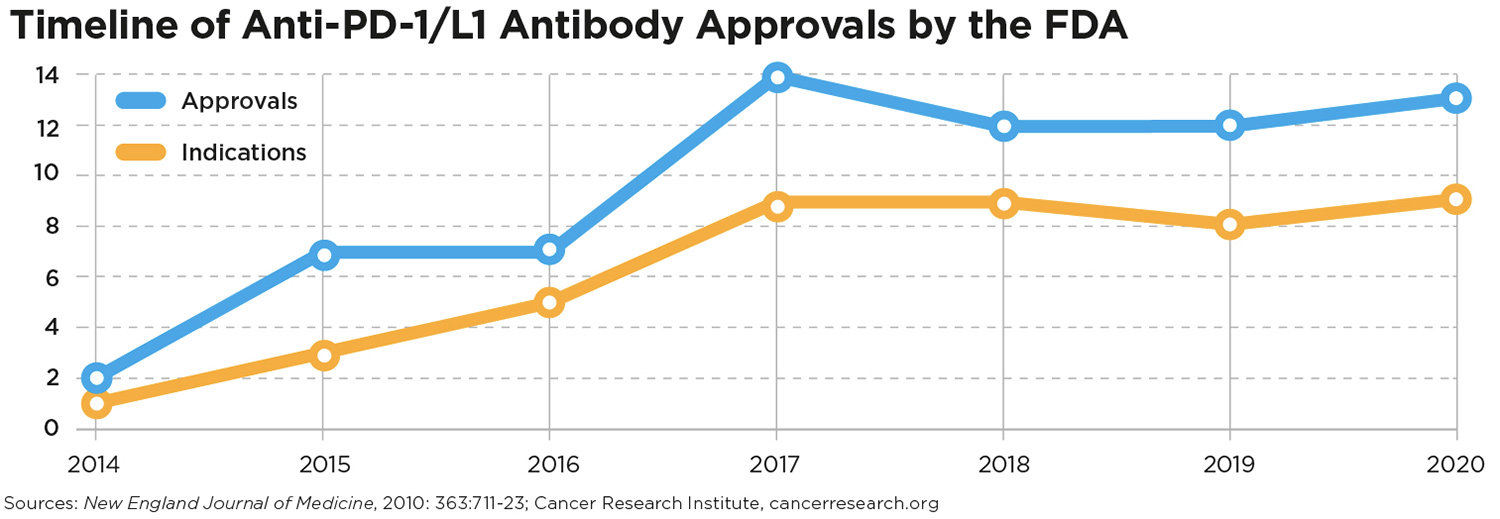
2017: The FDA expands indications for Keytruda (pembrolizumab)—which blocks PD-1—to include any metastatic or nonremovable solid tumor with certain molecular characteristics. This is the FDA’s first tissue/site-agnostic approval, meaning that the criteria for patient eligibility is based not on the location of the tumor, but on those molecular characteristics.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.




