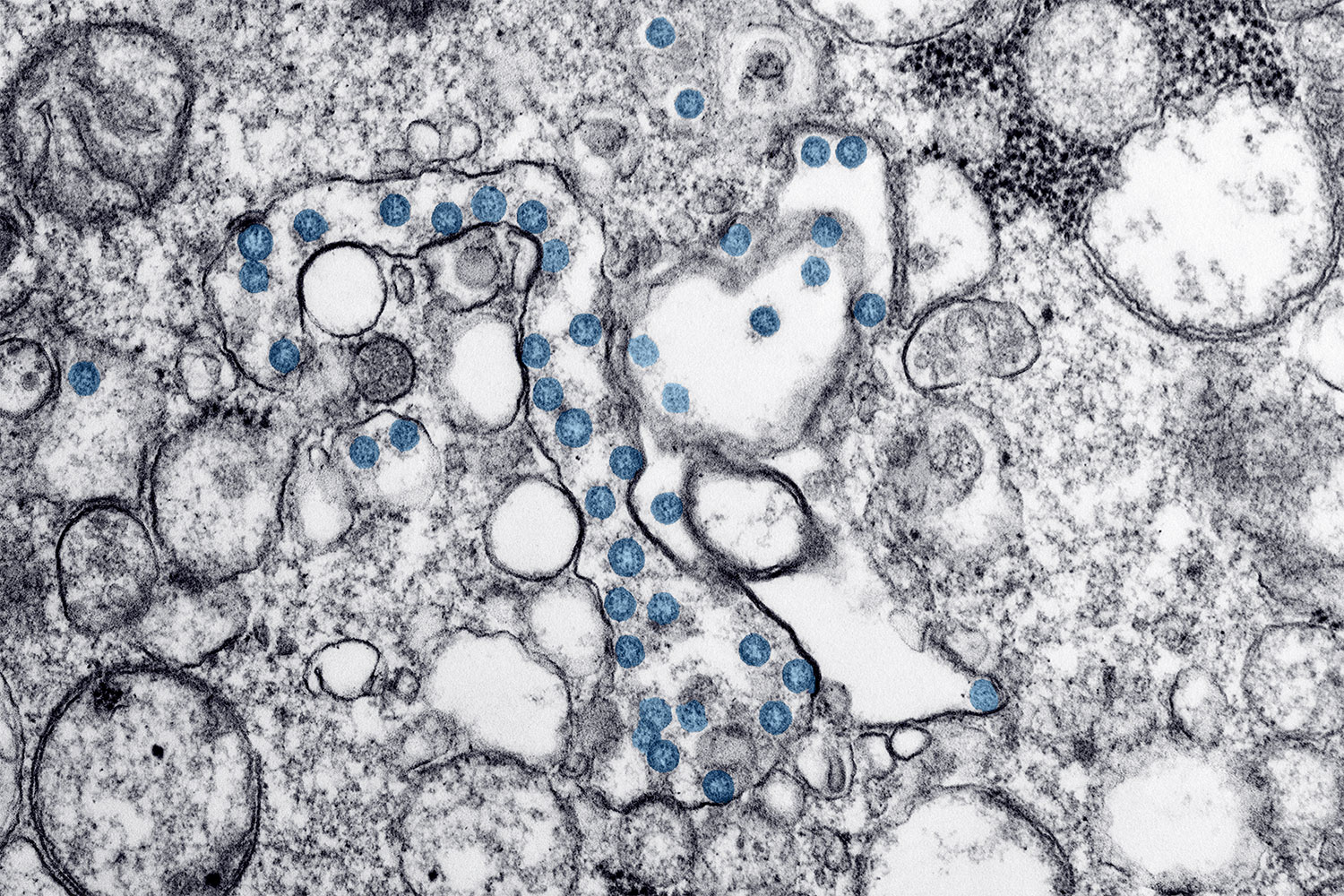
Cancer and the Novel Coronavirus
On March 11, the World Health Organization began to characterize the novel coronavirus, COVID-19, as a pandemic. In attempts to “flatten the curve”—meaning to slow transmission of the virus so that the health care system is not overwhelmed by cases all at once—some events are being canceled, some schools are closing and some companies are having their employees work from home. But what is known about how the virus is affecting cancer patients and survivors? Patients with blood cancers are believed to be at the highest risk of complications from COVID-19, infectious disease specialist Steve Pergam of the Fred Hutchinson Cancer Research Center in Seattle told the Washington Post. This is due both to the blood cancers themselves and immunosuppressive treatments that patients with these cancer types may undergo, including chemotherapy and bone marrow transplant. Patients receiving treatment for other cancer types are also at increased risk, especially those receiving chemotherapy but also those receiving other treatments. Oncologist and health policy expert Gary Lyman, also at Fred Hutchinson, said in an article by the Fred Hutch News Service that those no longer undergoing treatment may also want to use caution, as effects of treatment can be lasting. Cancer centers are taking various precautions, such as asking patients to call prior to coming in if they have certain symptoms, screening patients for respiratory symptoms at entrances, limiting visitors, and limiting travel, meetings and gatherings for staff. The Centers for Disease Control and Prevention is providing regular updates on COVID-19 on this page and offers recommendations for members of at-risk populations here.
Bias and Clinical Trial Enrollment
It’s known that members of racial and ethnic minority groups are underrepresented in cancer clinical trials. To explore bias and stereotyping as possible causes of this disparity, researchers interviewed 91 cancer center leaders, principal investigators, referring clinicians and research staff at five U.S. cancer centers about clinical trial recruitment. In a study published March 9 in Cancer, the researchers report that these medical and research professionals did exhibit signs of bias and stereotyping when it came to clinical trial recruitment. “Not only did some respondents view racial and ethnic minorities as less promising participants, some respondents reported withholding trial opportunities from minorities based on these perceptions,” the researchers write.
An Immunotherapy Combination for Liver Cancer
The Food and Drug Administration on March 10 approved the immune checkpoint inhibitors Opdivo (nivolumab) and Yervoy (ipilimumab) for treatment of patients with liver cancer. The combination is approved for patients who have previously been treated with Nexavar (sorafenib). Nexavar is indicated for treatment of patients whose liver cancer cannot be removed by surgery. Previously, Opdivo on its own and the checkpoint inhibitor Keytruda (pembrolizumab) on its own were each approved for liver cancer patients who had already taken Nexavar.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.