The Dangers of Staying Still
More sedentary behavior was associated with greater risk of death from cancer in a study of more than 8,000 Americans published June 18 in JAMA Oncology. Researchers asked the study participants to wear accelerometers for a week and assessed their movement levels. The participants had an average age of around 70 and were followed for an average of a little over five years. During this period, 268 participants died of cancer. The researchers calculated that engaging in an additional 30 minutes of light-intensity physical activity in place of sedentary behavior was associated with an 8% reduction in cancer mortality risk. Replacing 30 minutes of sedentary behavior with 30 minutes of moderate- to vigorous-intensity physical activity was associated with a 31% reduction in risk of death from cancer. In a press release, the researchers used cycling as an example of moderate-intensity activity and walking as an example of light-intensity activity.
Estimating the Consequences of COVID-19
Researchers at the National Cancer Institute (NCI) in Bethesda, Maryland, estimate that there will be more than 10,000 additional deaths from breast cancer and colorectal cancer in the U.S. in the next 10 years due to delays in screening, diagnosis and treatment related to COVID-19. The estimate, presented in an editorial published June 19 in Science, is conservative, writes NCI director Norman E. Sharpless, as it assumes moderate disruption to care lasting only six months. The estimate is based on a scenario in which only a quarter of patients undergo screening who were supposed to in these six months, and in which diagnosis and treatment are delayed for a third of patients, whether their disease was detected via screening or symptoms. “Ignoring life-threatening non–COVID-19 conditions such as cancer for too long may turn one public health crisis into many others. Let’s avoid that outcome,” Sharpless writes.
HPV Vaccine Approved for Prevention of Head and Neck Cancer
The Food and Drug Administration on June 12 approved the vaccine Gardasil 9 for prevention of head and neck cancers. Gardasil 9 prevents infection with nine types of the human papillomavirus (HPV). HPV is associated with development of cervical, vulvar, vaginal, anal and head and neck cancers, but the vaccine had until now only been approved for prevention of cervical, vulvar, vaginal and anal cancer. An article in STAT points out that the new approval does not change which people are recommended to get the vaccine. Gardasil 9 is approved for use in people ages 9 through 45. The new approval does mean that marketing materials on the vaccine can now mention head and neck cancer.
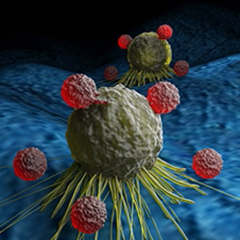
Immunotherapy Approved Based on Tumor Mutational Burden
As of June 16, the immune checkpoint inhibitor Keytruda (pembrolizumab) has been approved by the Food and Drug Administration (FDA) for treatment of people with advanced solid tumors who have high tumor mutational burden, regardless of cancer type, and no other satisfactory treatment options. Tumors are considered to have high tumor mutational burden if they have 10 or more mutations per million DNA base pairs. Tumors with many mutations may be more likely to excite an immune response. The FDA in 2017 approved Keytruda for treatment of patients whose tumors had defects in DNA repair that lead to genomic abnormalities, also regardless of cancer type.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.